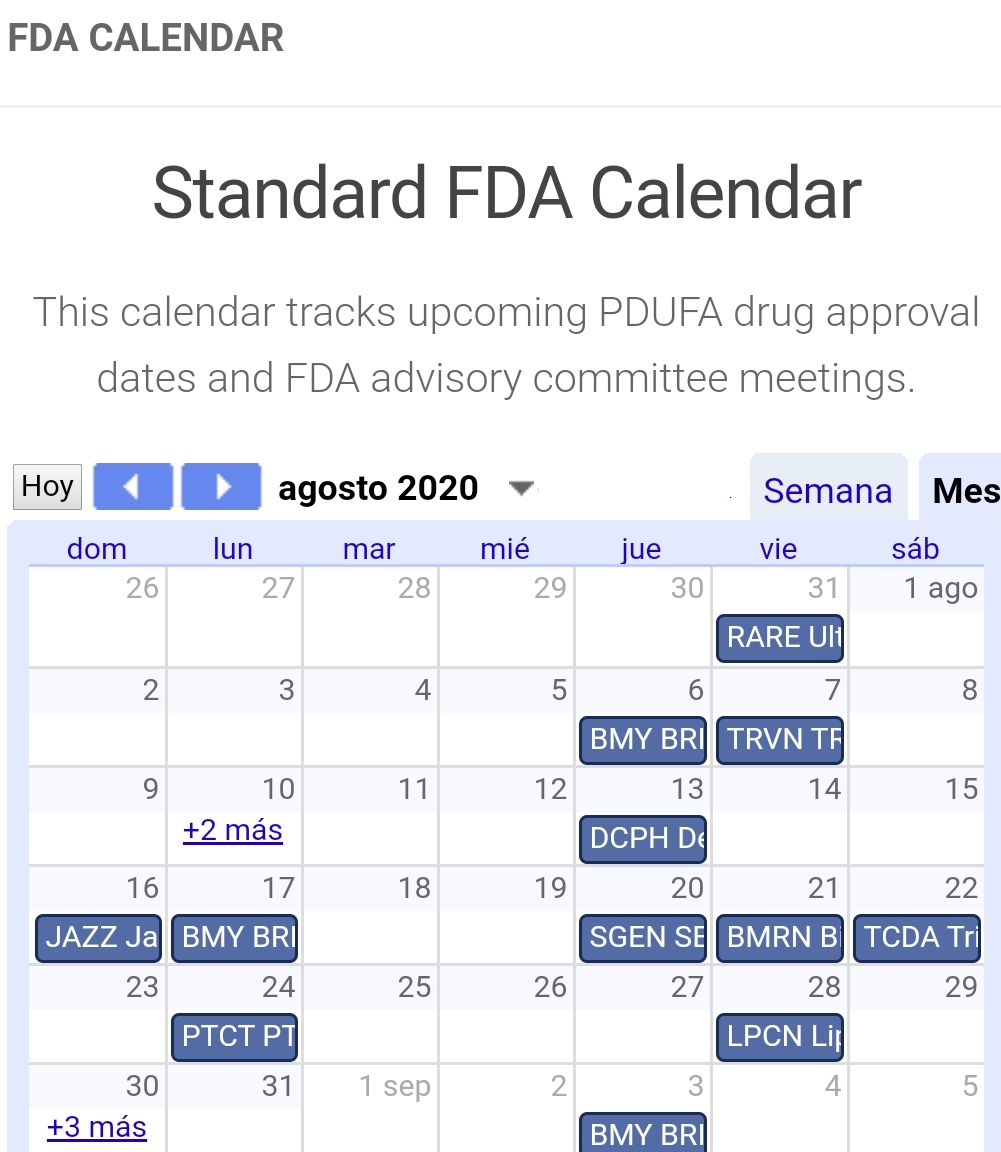
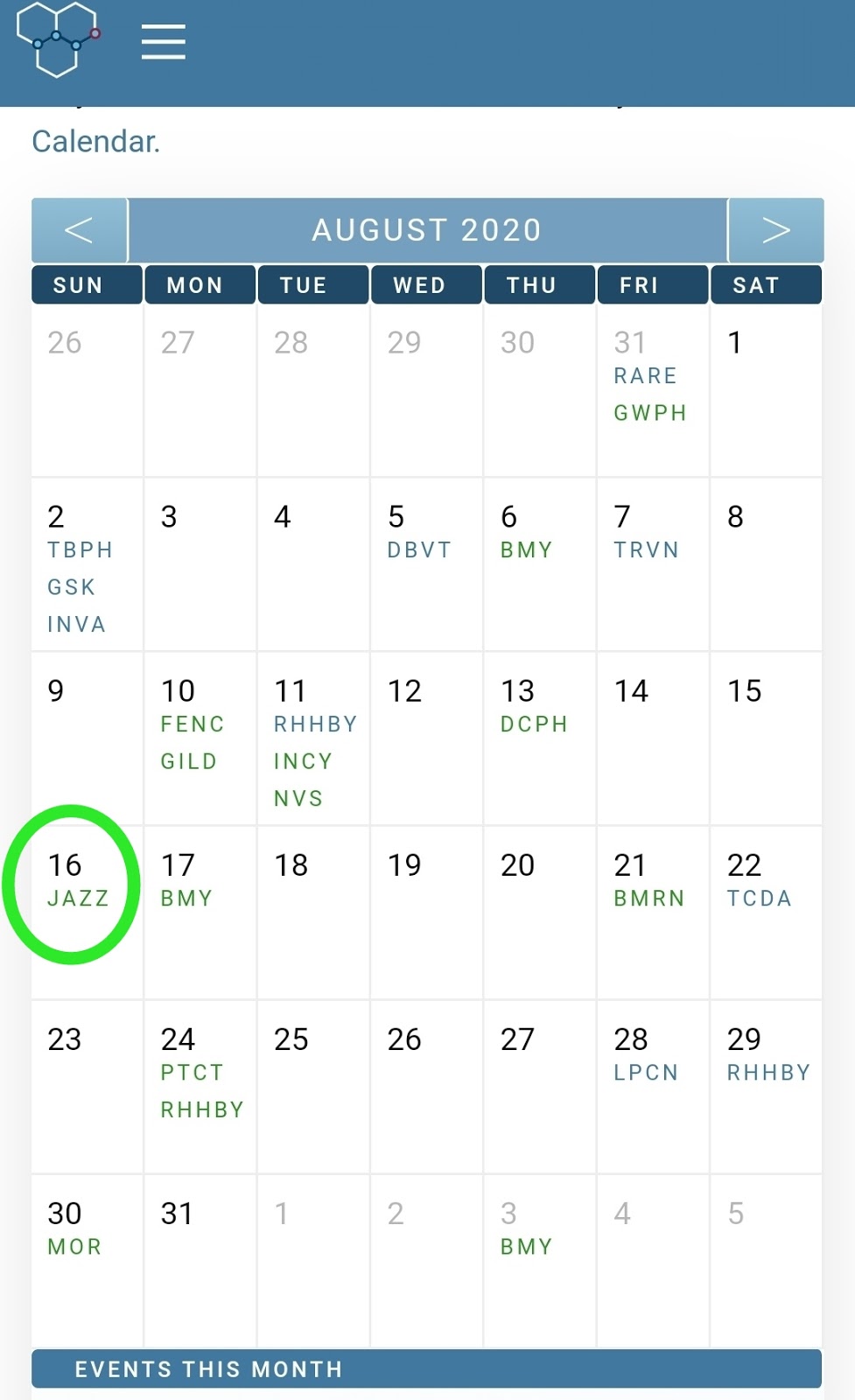
Fda Calendar Pdufa - Web what is a pdufa date? Pdufa target dates are dates by which the fda aims to deliver. Filing schedules for regulatory applications such as new drug application, or nda, supplemental nda, biologic license. Unlock more data with a free account. Web comprehensive suite of tools for trading and investing in biotech stocks. Web the pda/fda joint regulatory conference, celebrating its 33rd year of informing, educating, and guiding industry professionals and organizations on current good. The pdufa date refers to the date the food and. By establishing timelines and goals, it provides. The calendar will also provide key. Web our pdufa calendar includes future pdufa dates for biotech companies, as well as advisory committee meeting dates.
Navigate the FDA/PDUFA calendar, 50 Catalysts, and Options sentiments
The pdufa date refers to the date the food and. Web the fda pdufa report is a chronological table of pdufa target dates as well.
Fda Pdufa Calendar Customize and Print
Sponsors frequently publish pdufa dates for their pending. Web the food and drug administration held a virtual public meeting on july 23, 2020 to kick.
FDA/PDUFA Catalyst Calendar (*Updated) endMarch/April 2024 and Highest
Listing current months pdufa dates. Fda calendar, pdufa date calendar, biotech company screener and database and much more. Web fda calendar for drug catalysts for.
Your December FDA/PDUFA Calendar what will be on your list? (Updated
Web an fda calendar typically displays information about the expected timeline for a particular drug approval or pdufa date. Sponsors frequently publish pdufa dates for.
FDA/PDUFA Calendar for November and early December u/OkImplement5002
Web the federal food, drug, and cosmetic act (the fd&c act), as amended by the prescription drug user fee act of 2017 (pdufa vi), authorizes.
Fda Pdufa Calendar
The fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings (adcomm). Once the fda accepts a filing for.
FDA PDUFA Calendar, PDUFA Dates, FDA Approval Dates BiopharmIQ
Fda calendar, pdufa date calendar, biotech company screener and database and much more. Once the fda accepts a filing for the approval of a drug,.
Fda Pdufa Calendar Customize and Print
Web the pdufa date serves as a good first approximation of when a final decision on drug approval can be expected. By establishing timelines and.
SI GRÍFOLS NO QUIERE PASAR POR UNA JUNTA " BOCHORNOSA " MUCHO
The fda pdufa calendar is a chronological calendar of pdufa target dates as well as advisory meetings (adcomm). Web what is a pdufa date? Web.
Filing Schedules For Regulatory Applications Such As New Drug Application, Or Nda, Supplemental Nda, Biologic License.
Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion. Pdufa target dates are dates by. Fda periodically conducts meetings on the prescription drug user fee act (pdufa) program. By establishing timelines and goals, it provides.
Web What Is A Pdufa Date?
Sign up or log in to access our enhanced fda calendar! On august 18, 2017, the president signed into law the fda reauthorization act of 2017. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching, from the first new drug class for schizophrenia in. The calendar will also provide key.
Web The Pdufa Date Serves As A Good First Approximation Of When A Final Decision On Drug Approval Can Be Expected.
The pdufa date refers to the date the food and. Web the fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom). Web the pda/fda joint regulatory conference, celebrating its 33rd year of informing, educating, and guiding industry professionals and organizations on current good. Web pdufa dates, or in other words fda decision dates.
Web The Federal Food, Drug, And Cosmetic Act (The Fd&C Act), As Amended By The Prescription Drug User Fee Act Of 2017 (Pdufa Vi), Authorizes Fda To Assess And Collect Fees For.
The fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory. Sponsors frequently publish pdufa dates for their pending. Web under the terms of the agreement, agios will receive an upfront payment of $905 million upon approval of vorasidenib by the u.s. See more catalyst data with a free or premium account.