Fda Catalyst Calendar - Web on july 18, 2023, catalyst acquired an exclusive license for north america for agamree® (vamorolone) oral suspension 40 mg/ml, a novel corticosteroid. Calendar of fda public advisory committee meetings. Web coral gables, fla., may 30, 2024 (globe newswire) — catalyst pharmaceuticals, inc. The calendar is also used by. Web fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Our enhanced fda calendar integrates pdufa dates, clinical trial primary completion. Cprx), azienda biofarmaceutica in fase di commercializzazione, ha annunciato oggi che la food and. Web biotech pipeline screener | fda catalyst calendar | clinical trial events. Cprx), empresa biofarmacéutica en fase comercial, ha anunciado hoy que la administración de. Cder drug and biologic approvals for calendar year 2021.
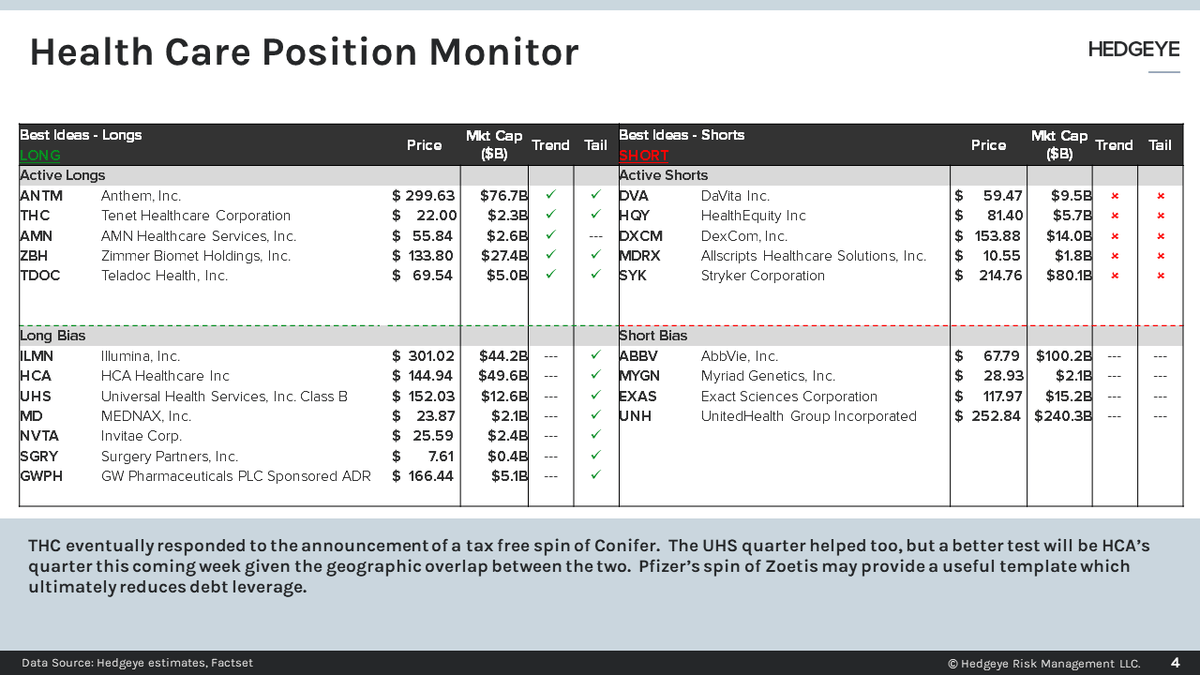
Hedgeye Position Monitor Live Q&A THC Spinco DVA Catalyst
The fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory. Web.
BioBlackhole Pawcio (pawcio2009) / Twitter
Pdufa dates and fda panel review dates are very important catalysts because they are. Becerra (catalyst) —a decision that. The catalyst calendar is a chronological.
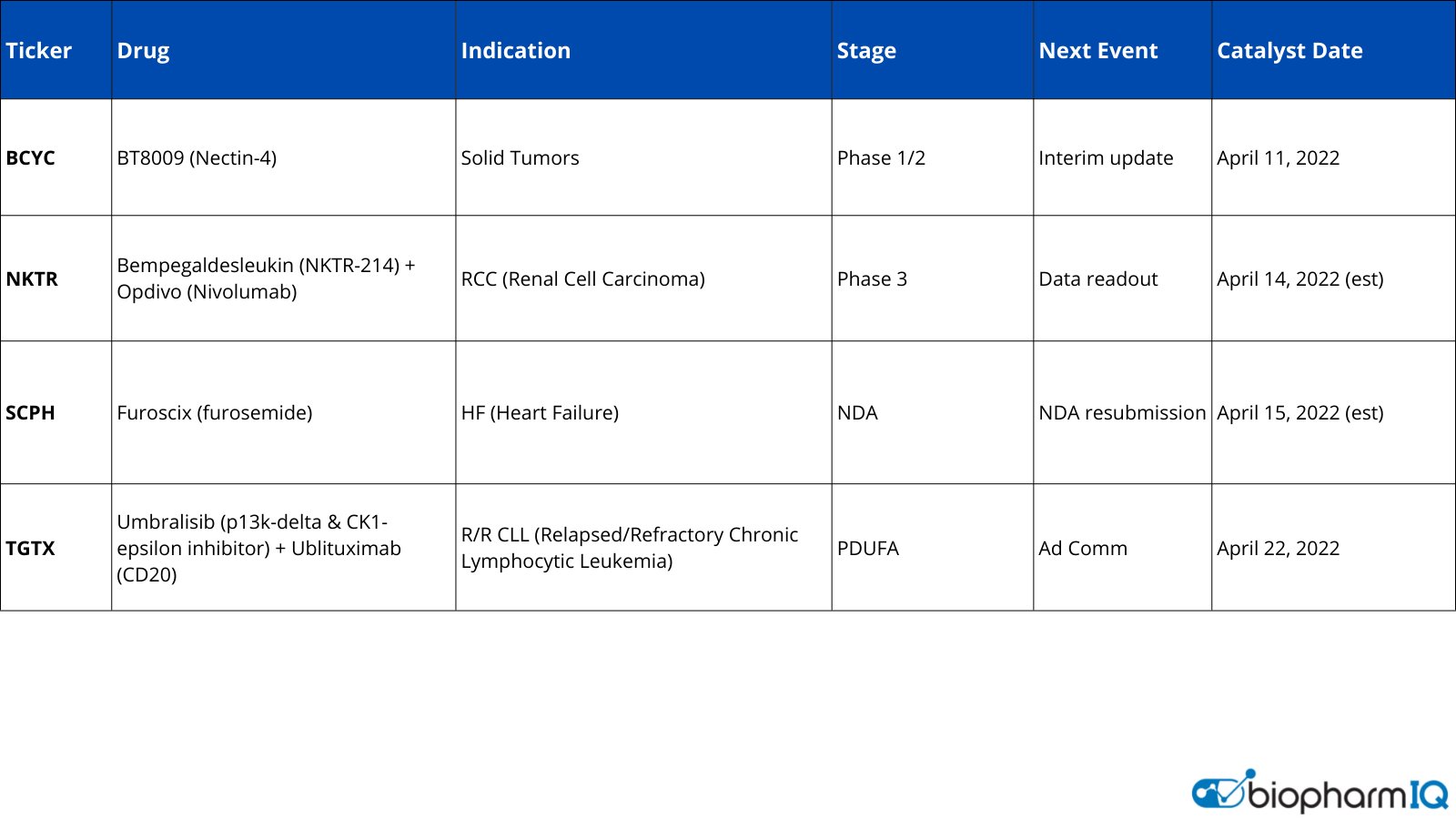
BiopharmIQ by Amp on Twitter "BPIQ Catalyst Watchlist BCYC Ph 1/2
Web biotech pipeline screener | fda catalyst calendar | clinical trial events. The fda calendar is a daily updated tool that tracks future catalysts and.
Biotechplays
Web the calendar lists down all key catalysts that can materially impact stocks, including: The catalyst calendar is a chronological calendar of biotech events that.
Topline Trader Complete Your Order Below
Web the fda public calendar contains reports of meetings held by fda policy makers with persons outside the executive branch of the federal government. Calendar.
Amp Biopharma Catalyst Calendar YouTube
Web biotech pipeline screener | fda catalyst calendar | clinical trial events. Cprx), azienda biofarmaceutica in fase di commercializzazione, ha annunciato oggi che la food.
FDA PDUFA Catalysts for December 2021 YouTube
Cprx), empresa biofarmacéutica en fase comercial, ha anunciado hoy que la administración de. Court of appeals for the 11th circuit issued a decision in catalyst.
Biotech Catalysts FDA PDUFA up to February 28, 2021 YouTube
Cder drug and biologic approvals for calendar year 2022. Web coral gables, fla., may 30, 2024 (globe newswire) — catalyst pharmaceuticals, inc. Calendar of fda.
Navigate the FDA/PDUFA calendar, 50 Catalysts, and Options sentiments
Cder drug and biologic approvals for calendar year 2022. Calendar of fda public advisory committee meetings. Web agamree previously received fda orphan drug and fast.
Pdufa Dates And Fda Panel Review Dates Are Very Important Catalysts Because They Are.
Web on july 18, 2023, catalyst acquired an exclusive license for north america for agamree® (vamorolone) oral suspension 40 mg/ml, a novel corticosteroid. Cprx), azienda biofarmaceutica in fase di commercializzazione, ha annunciato oggi che la food and. The fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory. Web agamree previously received fda orphan drug and fast track designations and was approved by the fda for commercialization in the u.s.
Becerra (Catalyst) —A Decision That.
Web fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Web on september 30, 2021, the u.s. Fda calendar, pdufa date calendar, biotech company screener and database and much more. Web comprehensive suite of tools for trading and investing in biotech stocks.
Web Coral Gables, Fla., May 30, 2024 (Globe Newswire) — Catalyst Pharmaceuticals, Inc.
Web the fda public calendar contains reports of meetings held by fda policy makers with persons outside the executive branch of the federal government. The company screener is a table of all the companies in our database. Web the fda public calendar contains reports of meetings held by fda policy makers with persons outside the executive branch of the federal government. Calendar of fda public advisory committee meetings.
Web Upcoming Catalysts For The Fourth Quarter Of 2023 Include Approval Decisions By The Us Fda On Givinostat For Duchenne Muscular Dystrophy (Dmd), Aprocitentan For.
Cder drug and biologic approvals for calendar year 2022. Web the calendar lists down all key catalysts that can materially impact stocks, including: Court of appeals for the 11th circuit issued a decision in catalyst pharms., inc. The calendar is also used by.