Pharmaceutical Fda Approval Calendar - Web filing schedules for regulatory applications such as new drug application, or nda, supplemental nda, biologic license application, or bla, supplemental bla, premarket. The fda’s 2024 pdufa calendar is far from complete, but already it contains. Web our enhanced fda calendar integrates pdufa dates, clinical trial primary completion dates, and working capital runway estimates into a single timeline that covers. After a down year, fda signs off on a bounty of new meds, including 7 from pfizer | fierce pharma. The fda approved 55 novel therapeutics in 2023, the second highest count in the past 30 years. Web the fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and pdufa. In the second quarter of 2023, the agency approved 13 new drugs (table 1), equalling the first quarter tally. Web agamree previously received fda orphan drug and fast track designations and was approved by the fda for commercialization in the u.s. Cder drug and biologic approvals for. Web agamree previously received fda orphan drug and fast track designations and was approved by the fda for commercialization in the u.s.
A Quick Overview on FDA Drug Approvals RAS LSS
Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies.
OnePager Express FDA Approval Timeline Made in Excel with Project
Listen to the article 5 min. (teva), said that the u.s. Web update 22 january 2024. Food and drug administration has approved austedo. Web agamree.
How drugs are approved DEBRA UK The butterfly skin charity
Web get daily updates on important fda approval, pdufa dates, and fda advisory committee meetings with rttnews fda calendar & upcoming approvals. Innovative drugs often.
2022 FDA Drug Approval List, 2022 Biological Approvals and Approved
The fda approved 55 novel therapeutics in 2023, the second highest count in the past 30 years. Web on august 10, 2022, the food and.
New Drug Approvals & FDA Approvals 2021 Huateng Pharma
(teva), said that the u.s. Cder drug and biologic approvals for. Web our fda calendar is designed to provide you with future catalysts across biotech.
Fda Approval Calendar 2023 Everything You Need To Know August 2023
Web the fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory.
FDA Drug Approval Process Infographic (Horizontal) FDA
Web on august 10, 2022, the food and drug administration granted regular approval to capmatinib (tabrecta, novartis pharmaceuticals corp.) for adult patients with. Oncology (cancer).
New Drugs Approvals by FDA and EMA 2020 Recap Pharma Excipients
Web update 22 january 2024. Web fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates..
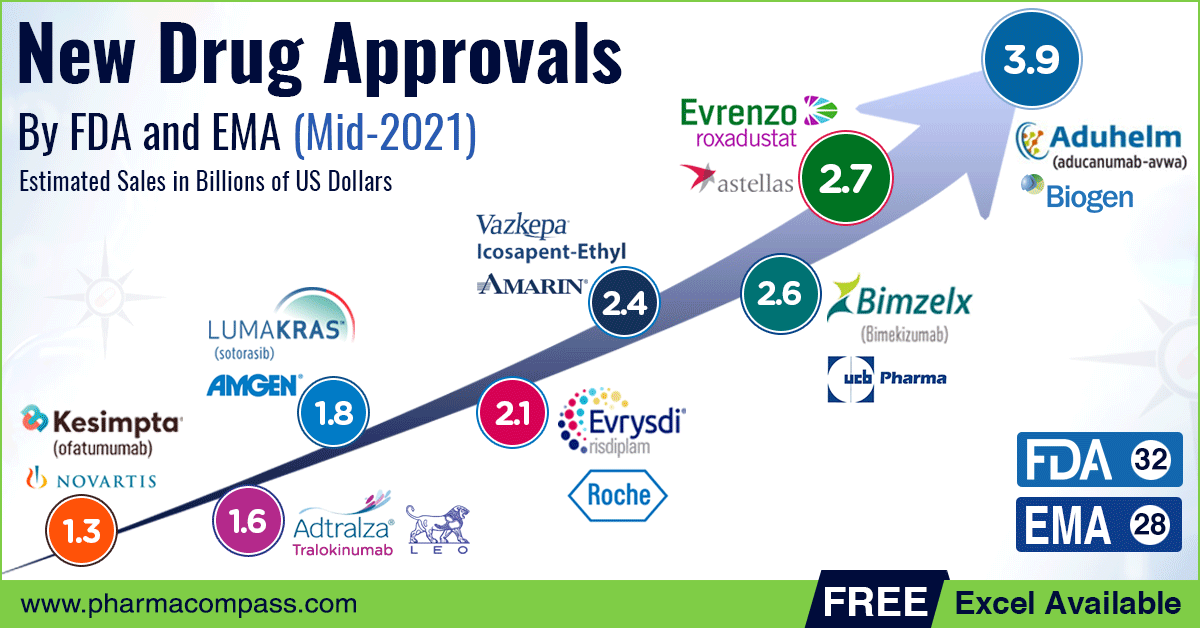
New Drug Approvals by FDA and EMA Mid2021 Recap Radio Compass Blog
Listen to the article 5 min. Affiliate of teva pharmaceutical industries ltd. After a down year, fda signs off on a bounty of new meds,.
Web Update 22 January 2024.
Web fda calendar for drug catalysts for approvals/crls, advisory committee meetings and phase 1,2 & 3 trial data release dates. Web our fda calendar is designed to provide you with future catalysts across biotech & pharma companies, updated on a daily basis for all companies we cover. (teva), said that the u.s. Web filing schedules for regulatory applications such as new drug application, or nda, supplemental nda, biologic license application, or bla, supplemental bla, premarket.
Web Our Enhanced Fda Calendar Integrates Pdufa Dates, Clinical Trial Primary Completion Dates, And Working Capital Runway Estimates Into A Single Timeline That Covers.
The fda’s 2024 pdufa calendar is far from complete, but already it contains. Food and drug administration has approved austedo. Innovative drugs often mean new. Web the fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and pdufa.
Web Get Daily Updates On Important Fda Approval, Pdufa Dates, And Fda Advisory Committee Meetings With Rttnews Fda Calendar & Upcoming Approvals.
The fda approved 55 novel therapeutics in 2023, the second highest count in the past 30 years. Cder drug and biologic approvals for calendar year 2021. After a down year, fda signs off on a bounty of new meds, including 7 from pfizer | fierce pharma. In the second quarter of 2023, the agency approved 13 new drugs (table 1), equalling the first quarter tally.
The Fda Approved 50 Novel Drugs In 2021, Including The First Kras Inhibitor For Cancer And The First Anti.
Web below is a listing of new molecular entities and new therapeutic biological products approved by cder and organized by calendar year. Affiliate of teva pharmaceutical industries ltd. Listen to the article 5 min. Cder drug and biologic approvals for calendar year 2022.